By Faith Nyasuguta
The World Health Organization has endorsed the widespread use of the first-ever Malaria vaccine in Africa.
Experts say that the move could annually save tens of thousands of children’s lives across the continent.
Dubbing the move “historic”, the WHO director-general, Dr. Tedros Adhanom Ghebreyesus, said that after a triumphant pilot programme in three African nations, the RTS, S vaccine ought to be made available more widely.
“I started my career as a malaria researcher, and I longed for the day that we would have an effective vaccine against this ancient and terrible disease. And today is that day, a historic day. Today, the WHO is recommending the broad use of the world’s first malaria vaccine,” Tedros revealed at a Geneva, Switzerland press conference.
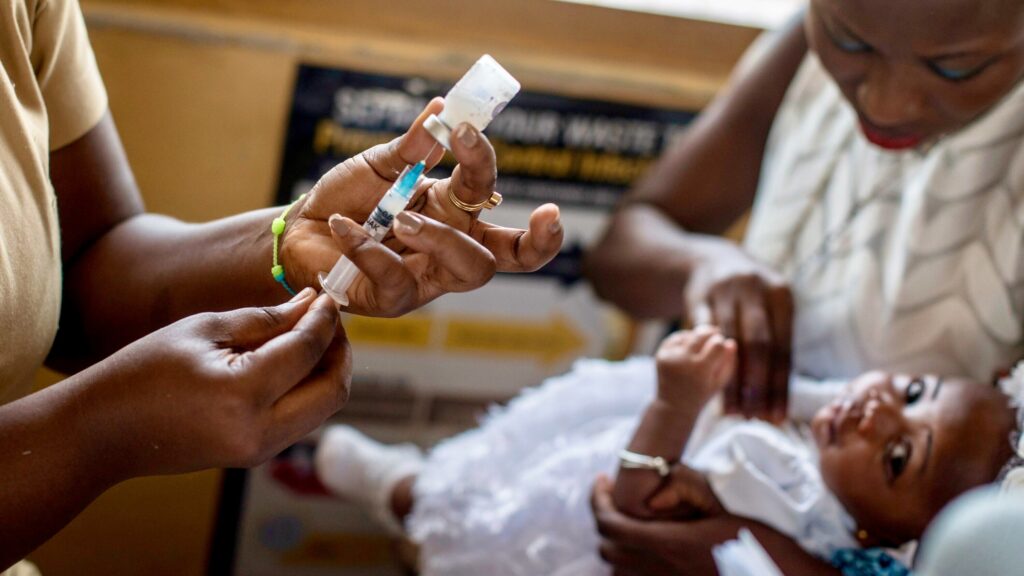
The RTS, S jab, alias Mosquirix, was developed by the British pharmaceutical company GlaxoSmithKline (GSK) and has so far been administered to over 800,000 children in Ghana, Kenya, and Malawi since the pilot programme launched in 2019.
The vaccine has undergone lengthy clinical trials and has limited efficacy. It prevents just 39 per cent of Malaria cases and 29 per cent of severe Malaria cases among young African children above four years.
Despite that, an August study spearheaded by the London School of Hygiene & Tropical Medicine (LSHTM) revealed that when the children were given both the RTS,S and antimalarial drugs, there was a 70 per cent drop in hospitalization or death.
“Using this vaccine in addition to existing tools to prevent malaria could save tens of thousands of young lives each year,” Tedros said on Wednesday.
“It is safe. It significantly reduces life-threatening, severe malaria, and we estimate it to be highly cost-effective.”
He added: “Malaria has been with us for millennia, and the dream of a malaria vaccine has been a long-held, but unattainable dream. Today, the RTS, S malaria vaccine, more than 30 years in the making, changes the course of public health history. We still have a very long road to travel. But this is a long stride down that road.”
For a while now, there have been fears that years of progress towards bringing a halt to malaria has stalled, with some nations, including Eritrea and Sudan, experiencing key reappearances in recent years.
In 2019, some 409,000 people, mostly in Africa, died from Malaria with over 270,000 of the victims being children under five.
However, experts believe that the WHO’s endorsement will re-energize the race to find other vaccines, a quest that has been ongoing for about 100 years.
Earlier in 2021, scientists at the Jenner Institute of Oxford University revealed that a vaccine they had developed had shown results that would make it the first to meet the WHO goal of 75 per cent efficacy.
For over a year, the jab showed up to 77 per cent efficacy in a trial in Burkina Faso involving some 450 children. Larger trials are now kicking off with 4,800 children in four nations.
GSK’s chief global health officer, Thomas Breuer, said: “GSK is proud that RTS, S, our groundbreaking malaria vaccine, developed over decades by our teams and partners, can now be made available to children across sub-Saharan Africa.”
“This long-awaited landmark decision can reinvigorate the fight against malaria in the region at a time when progress on malaria control has stalled. Both real-world evidence and clinical trial data show that RTS, S, alongside other malaria prevention measures, has the potential to save hundreds of thousands of lives.”
GSK reassured its commitment to supplying up to 15 million doses annually at a price not exceeding five per cent more than the production cost. It is set to work alongside partners, funders, and states to support the additional supply of the vaccine.




